A Guide to Implementing ISO 9001
ISO 9001:2015 is an international standard for Quality Management Systems, ensuring organisations consistently meet customer and regulatory requirements while enhancing customer satisfaction.
It provides a framework for effective process management and continuous improvement. Widely adopted across various industries, ISO 9001 promotes operational excellence and efficiency.
Below, we have provided an overview of the contents covered in our ISO 9001 Implementation Guide PDF.
Why ISO 9001 matters
ISO 9001, part of the ISO 9000 family, is the international standard for QMS. Established by the International Organisation for Standardization (ISO), its primary goal is to ensure that organisations consistently provide products and services that meet customer and regulatory requirements, while also enhancing customer satisfaction through effective system application.
A brief history of ISO 9001
First published in 1987, ISO 9001 evolved from the British Standard BS 5750 series, which was heavily influenced by government and military procurement standards designed to ensure the quality of products and services.
Over the years, ISO 9001 has undergone several revisions to stay relevant and address the changing needs of industries and markets. The most recent major update was in 2015, which introduced a greater emphasis on risk management, leadership involvement, and a more flexible approach to documentation.
Additionally, the latest revisions (February 2024) have incorporated considerations for climate change, highlighting the importance of sustainable practices and the impact of environmental factors on quality management systems. These updates reflect the growing awareness and need for businesses to adapt to and mitigate the effects of climate change while maintaining high standards of quality.
Benefits of implementing ISO 9001:
Implementing ISO 9001 offers numerous advantages that can significantly enhance an organisation's performance and market position. Below are just a few of the benefits:
-
Enhanced customer satisfaction and loyalty: adopting a customer-focused approach adds value for customers, increasing their satisfaction and loyalty, leading to more repeat business.
-
Improved reputation: demonstrating a formal commitment to quality through a QMS positively impacts your reputation and is often a prerequisite for formal tendering procedures, especially for public sector contracts.
-
Increased revenue and market share: a certified QMS can open doors to a range of contract opportunities, potentially boosting revenue and market share.
-
Operational efficiency: implementing a QMS helps use resources—people, materials, time, money, and external partners - more effectively and efficiently, directly enhancing profitability.
-
Effective problem solving: focusing on root cause analysis ensures robust solutions and effective improvements.
What is inside the ISO 9001 Implementation Guide?
Our PDF guide for Implementing ISO 9001 is a valuable resource for organisations seeking certification. Below you will find an overview of each key section within the guide.
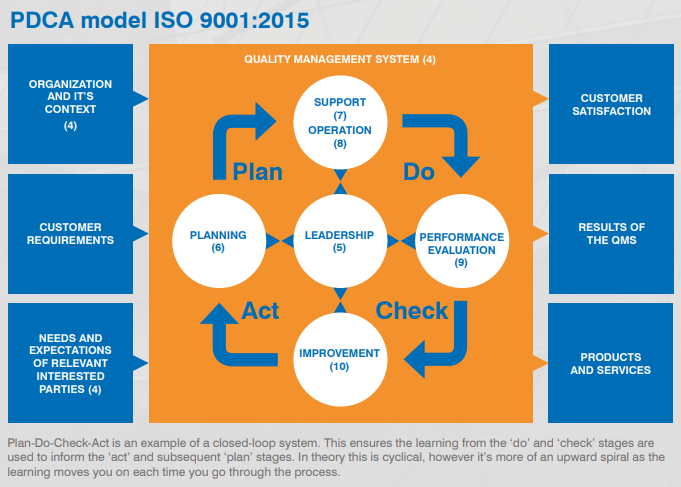
PDCA Cycle
ISO 9001 is based on the Plan-Do-Check-Act (PDCA) cycle, also known as the Deming wheel or Shewhart cycle. This iterative method is crucial for continuous improvement. The cycle can be applied to the entire QMS or individual elements, ensuring a persistent focus on enhancing processes and outcomes.
See the PDCA model here:
Risk-Based and Process-Based Thinking Audits
Risk-based and process-based thinking are central to ISO 9001. They involve identifying, assessing, and managing risks that could impact the QMS.
Audits are systematic, evidence-based evaluations of your Quality Management System (QMS) to verify its effectiveness and compliance with ISO 9001 standards. They embody risk-based thinking, focusing on processes with higher risks.
A process is the transformation of inputs to outputs, which takes place as a series of steps or activities which result in the planned objective(s). Often the output of one process becomes an input to another subsequent process. Very few processes operate in isolation from any other.
Using a process approach in auditing ensures thorough and effective evaluations, supporting the continuous enhancement of your QMS.
Annex SL
Prior to the adoption of Annex SL there were many differences between the clause structures, requirements and terms and definitions used across the various management system standards.
Annex SL is a framework adopted by ISO 9001 to provide a unified structure for ISO management system standards, including ISO 14001 and ISO 45001. This standardisation facilitates easier integration of multiple ISO standards within an organisation.
Annex SL consists of 10 key clauses:
-
Scope: defines the boundaries and applicability of the QMS.
-
Normative references: lists referenced documents necessary for application, such as ISO 9000:2015.
-
Terms and definitions: clarifies key terms like 'product', 'process', and 'interested parties'.
-
Context of the organisation: adopts a holistic approach to customer focus, defining internal and external contexts through SWOT analysis.
-
Leadership: emphasises active leadership involvement in the QMS.
-
Planning: focuses on preventive action, outlining what needs to be done, resources required, responsibilities, timelines, and methods.
-
Support: covers necessary resources, including people, infrastructure, environment, knowledge, and communication.
-
Operation: the 'Do' stage, ensuring appropriate control over the creation and delivery of products/services.
-
Performance evaluation: evaluates QMS performance through process monitoring, customer feedback, internal audits, and management review.
-
Improvement: uses monitoring and measurement data to enhance processes and customer satisfaction.
Get the most from your management system
This section of the Implementation Guide offers practical advice to help you optimise your Quality Management System (QMS). The goal is not just to meet ISO 9001 standards but to enhance overall business performance and customer satisfaction.
Key recommendations include aligning your QMS with strategic objectives to ensure long-term relevance, engaging all employees to foster a culture of quality, and setting SMART objectives to drive effective improvements.
The guide also highlights the importance of tailoring risk assessment methods to your specific needs and securing strong commitment from top management to support and integrate the QMS effectively.
By following these insights, you can create a more robust and effective QMS that supports continuous improvement and organisational success.
Next steps once implemented
Implementation is not the end but the beginning of a continuous journey. Post-implementation, organisations should:
-
Regularly review and update the QMS.
-
Conduct periodic audits.
-
Engage in ongoing training and development.
-
Seek continual feedback and improvement opportunities.
NQA’s final thoughts
Reading our Implementation Guide will enhance your readiness for ISO 9001 implementation and certification. This comprehensive PDF offers in-depth insights and practical advice, making it an essential resource for those dedicated to upholding and advancing your quality management system.
Access the full ISO 9001:2015 Implementation Guide here.
![]() Want to gain more knowledge on ISO 9001? Explore our latest training!
Want to gain more knowledge on ISO 9001? Explore our latest training!
can support you.